Advancing Cancer Care: The Emergence of Antibody Drug Conjugates (ADCs)
Precision Oncology: A New Era in Targeted Cancer Treatment
For decades, chemotherapy has served as a cornerstone in cancer therapy, saving countless lives globally. Though, its lack of selectivity frequently enough results in collateral damage too healthy cells, causing notable side effects that impact patients’ quality of life.
Recently, antibody drug conjugates (ADCs) have gained momentum as a transformative option. By merging the specificity of antibodies with powerful cytotoxic agents, ADCs deliver treatment directly to malignant cells while sparing normal tissues-offering hope for more effective adn less toxic cancer therapies.
the Mechanism Behind ADCs: Combining Targeting Accuracy with Therapeutic Strength
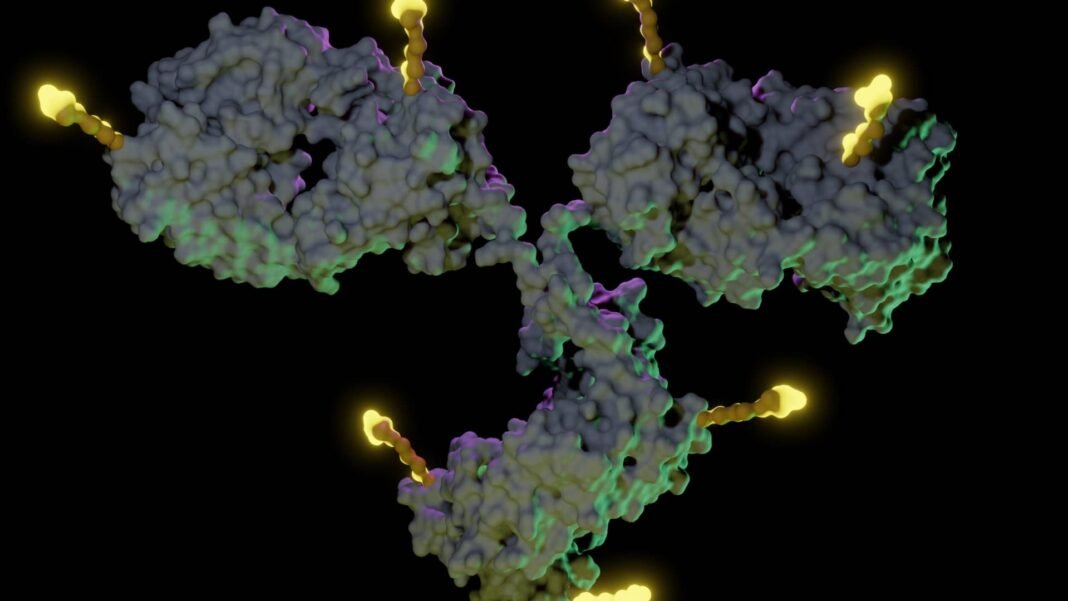
An ADC consists of three essential components: a monoclonal antibody that recognizes tumor-specific antigens; a potent cell-killing payload; and a chemical linker that attaches the two and controls drug release timing. This design enables concentrated delivery of chemotherapy agents at tumor sites while minimizing systemic exposure.
Next-generation adcs such as Enhertu-developed by AstraZeneca and Daiichi Sankyo-have optimized this platform by increasing the amount of cytotoxic payload delivered per dose and utilizing sophisticated linkers that activate only within the tumor microenvironment.Notably, Enhertu’s capacity to target neighboring cancer cells with low HER-2 expression exemplifies advances in overcoming tumor heterogeneity.
The Expanding Market Landscape for Antibody Drug Conjugates
The global oncology market is witnessing rapid growth driven by ADC innovation. Projections estimate that by 2028,ADC sales will reach nearly $31 billion within an overall $375 billion oncology sector-a clear indicator of their rising clinical importance.
This surge has attracted major pharmaceutical players including Pfizer, Merck, AbbVie, Bristol Myers Squibb (BMS), Eli Lilly, Johnson & Johnson (J&J), Gilead Sciences, GlaxoSmithKline (GSK), and astrazeneca investing heavily into research and growth. Pfizer’s landmark acquisition of Seagen for $43 billion highlights industry confidence in the future potential of these therapies.
Key Milestones Demonstrating Clinical Success
- AstraZeneca’s Enhertu: Approved for breast, lung, and gastric cancers; generated over $3.7 billion in 2024 sales with clinical trials showing it extends progression-free survival beyond one year compared to standard chemotherapy in HER-2-positive metastatic breast cancer patients.
- Pfizer’s Adcetris: Utilized as first-line treatment combined with chemotherapy for certain lymphomas; reported revenues close to $1.1 billion last year.
- Pfizer/astellas’ Padcev: Approved alongside Merck’s Keytruda for initial bladder cancer therapy; achieved $1.69 billion revenue during 2024.
- Gilead’s Trodelvy: When paired with Keytruda against aggressive triple-negative breast cancers expressing PD-L1 protein showed a 35% reduction in disease progression risk; earned approximately $1.3 billion despite earlier trial challenges this year.
Tackling Challenges: Improving Safety Profiles and Therapeutic Precision
The path toward ideal ADC treatments involves overcoming several obstacles:
- Cytotoxic payload leakage can cause unintended toxicity affecting healthy tissues;
- Selecting highly specific tumor antigens remains critical yet complex;
- Diverse genetic profiles across tumors lead to variable responses;
- Dosing regimens must carefully balance efficacy against tolerability concerns;
The pharmaceutical industry is addressing these issues through innovations such as advanced linker chemistries ensuring controlled drug release exclusively inside tumors and exploring novel cytotoxins like targeted protein degraders designed to bypass resistance mechanisms encountered by conventional agents.
Learnt Insights from Past Setbacks
- Bristol Myers Squibb discontinued an HER-3 targeting ADC after failing to improve overall survival despite slowing tumor growth;
- AstraZeneca withdrew Blenrep globally due to safety concerns but later reintroduced it following dosing modifications enhancing tolerability;
- Trodelvy experienced early trial failures but demonstrated renewed promise when combined strategically with immune checkpoint inhibitors enhancing anti-tumor immunity;
Pioneering Innovations Among Industry Leaders
AbbVie broke new ground by securing approval for the first c-Met targeting ADC aimed at non-small cell lung cancers known for poor outcomes while advancing candidates against SEZ6 proteins prevalent in neuroendocrine tumors like small-cell lung carcinoma-with response rates two-to-three times higher than traditional chemotherapies observed during clinical evaluations.
Bristol Myers Squibb is developing bispecific antibodies capable of simultaneously binding EGFR and HER-3 receptors-enhancing selectivity while reducing adverse events such as interstitial lung disease common among older treatments.This dual-target approach aims at maximizing therapeutic benefit through synergistic engagement mechanisms currently undergoing late-stage trials notably focused on triple-negative breast cancers.
Eli Lilly integrates cutting-edge linker technologies acquired via Mablink acquisition enabling prolonged circulation time coupled with improved penetration into solid tumors demonstrated recently using folate receptor alpha-targeted candidates aimed at ovarian malignancies-with preliminary data indicating reduced ocular toxicities compared to earlier generations.
Johnson & Johnson (J&J) leverages expertise around prostate-specific membrane antigen (PSMA)-a marker abundant on prostate tumors yet untapped by approved ADCs-and combines stable linker platforms alongside companion diagnostics facilitating precise patient selection ahead of imminent clinical trials.
Synchronized Strategies: Merging Antibody Drug Conjugates With Immunotherapy Agents
Cancer experts predict chemotherapy will continue playing a role within multi-modal regimens but foresee growing adoption of combination approaches pairing true precision medicines like antibody drug conjugates synergistically combined with immune checkpoint inhibitors or T-cell engagers over coming years.This method exploits complementary actions where ADCS induce immunogenic cell death activating immune surveillance while checkpoint blockers unleash full immune system potential-significantly improving outcomes including progression-free survival rates documented across recent major oncology conferences worldwide......
A prime example includes Pfizer deploying vedotin-based platforms successfully paired so far with Keytruda targeting integrin B6 or PD-L1 proteins expressed across various solid tumors including non-small cell lung cancers-with pivotal phase III studies planned later this year based on encouraging early efficacy signals demonstrating superior response rates versus monotherapies alone.
BioNTech pursues similar bispecific antibody-drug combinations aiming not only replace conventional chemotherapies but also enhance existing immunotherapeutics blocking PD-1 pathways-their pipeline features four distinct investigational molecules undergoing rigorous testing phases.
Simultaneously J&J prepares groundbreaking studies combining proprietary PSMA-targeted adcs alongside T-cell engager immunotherapies designed explicitly recruit immune effectors directly towards malignant prostate tissue offering hope toward durable remissions previously unattainable.
The Road Ahead: Shaping Future Cancer Therapies With Antibody Drug Conjugates
The rapid evolution seen within antibody drug conjugate technology signals profound shifts poised to redefine oncologic care over the next decade.
While challenges remain regarding optimizing target specificity,safety margins,and broad applicability,the accelerating pace fueled by robust investments coupled emerging scientific breakthroughs promises increasingly personalized options tailored precisely according individual patient tumor biology.
As refined combination regimens integrating targeted cytotoxics plus immune modulators emerge,the vision brightens where many patients may avoid harsh systemic chemotherapies altogether achieving longer lives marked quality.Ultimately,this new frontier heralds hope grounded firmly upon molecularly guided interventions transforming standards-of-care worldwide placing personalized medicine front-and-center battling one humanity’s most formidable diseases today.