Canada Launches Groundbreaking Oral HIV Self-Test for At-Home Screening
Health Canada has recently authorized a novel, non-invasive oral HIV self-test, enabling Canadians to conveniently check their HIV status from home without the need for clinical visits.
Fast, Blood-Free Testing with Immediate Results
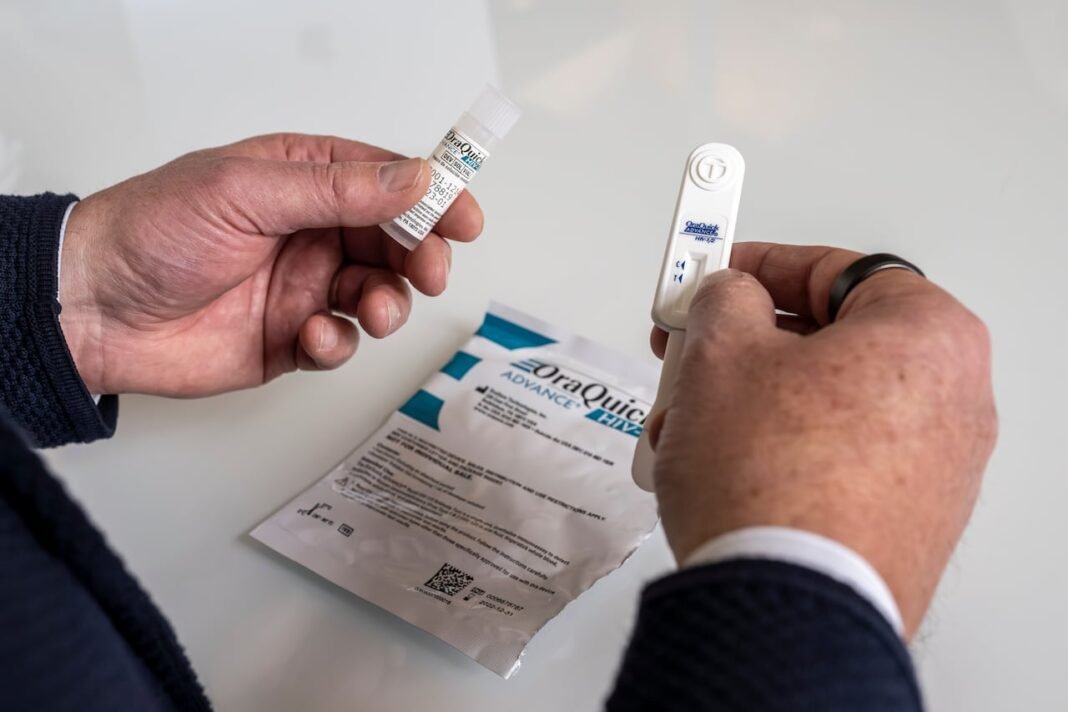
The OraQuick HIV self-test, created by U.S.-based OraSure Technologies adn now available in Canada through Toronto’s MAP Center for Urban Health Solutions at St. Michael’s Hospital, delivers results in about 20 minutes using only a saliva swab. This is the first oral-based HIV test approved nationwide that removes the requirement of finger-prick blood samples.
Orders can be placed online promptly with shipments expected to begin shortly after purchase.
Addressing Canada’s Shifting HIV Diagnosis Trends
Recent surveillance data shows variability in new HIV diagnoses across Canada: after reaching a peak of 2,434 cases in 2023-up from approximately 1,800 cases in 2022-the number dropped again to near 1,826 new infections reported so far in 2024. However, provinces such as Manitoba and Saskatchewan continue to report significantly higher infection rates (19.5 and 18.6 per 100,000 people respectively) compared to the national average of just under six per 100,000.
This innovative oral testing option targets individuals who may avoid conventional healthcare settings or feel uneasy about blood-based tests-estimated at around 7,000 Canadians living with undiagnosed HIV infections.
Focusing on Vulnerable Populations
The introduction of this easy-to-use test is especially valuable for groups facing elevated risk levels including racialized communities, men who have sex with men (MSM), and people who inject drugs. By offering a discreet method that can be performed privately at home or other non-clinical locations, it helps close gaps where conventional testing methods often fall short.
The Science Behind OraQuick: How it Detects HIV Antibodies
The device resembles a small stick equipped with an absorbent pad designed specifically for swabbing both upper and lower gums-areas rich in antibodies produced early after exposure to HIV. After collecting saliva via this swab technique, users insert it into a plastic cartridge where results develop over roughly twenty minutes.
- A single control line confirms the test worked properly but no infection was detected (negative result).
- A second line appearing alongside indicates presence of antibodies consistent with an HIV-positive result.
According to health authorities like the U.S. Food and Drug Management (FDA), antibody tests such as OraQuick typically detect infections within three weeks up to three months post-exposure-a window period during which antibodies reach detectable levels either through saliva or blood samples alike.
Reliability & Emotional Considerations post-testing
Research demonstrates that rapid oral fluid tests maintain accuracy comparable to laboratory diagnostics; however confirmatory laboratory blood testing remains essential following any positive result due to possible false positives or rare inconsistencies inherent even among highly sensitive assays.
Mental health professionals highlight that receiving unexpected positive results can cause notable emotional distress; therefore each kit includes comprehensive educational materials outlining next steps along with counseling resources and linkage-to-care options tailored toward immediate support needs regardless of outcome status.
User Experiences & Community Benefits

Mental health workers embedded within marginalized populations report strong enthusiasm surrounding these less invasive kits compared with earlier finger-prick alternatives-which some found intimidating due primarily to handling their own blood samples.
One community advocate observed increased willingness among those reluctant about engaging formal healthcare systems when offered accessible self-testing options free from needles-highlighting potential breakthroughs toward reducing undiagnosed cases nationwide through expanded outreach supported by these tools.
Pricing between $15-$20 per unit makes them relatively affordable but still potentially cost-prohibitive without subsidies especially among youth facing economic challenges intensified by inflationary pressures on living costs across Canada today.
Advocates call for coordinated government funding initiatives aimed at subsidizing costs further or providing free distribution channels targeting vulnerable populations most affected by ongoing transmission risks.
an Established Global Solution Now Available Locally
- OraQuick received FDA approval back in 20121;
- The World health Organization endorsed its global use starting mid-2016;
- Tens of millions have utilized similar kits worldwide spanning over sixty countries;
Navigating Funding & Distribution Obstacles Ahead
Although federal investments previously supported finger-prick kit rollouts-including $8 million allocated between late-2021 through early-2024-no dedicated funding currently exists specifically earmarked for distributing newer oral tests nationally.
Provincial programs recently launched within Saskatchewan and Nova Scotia offer promising models combining public financing alongside nonprofit partnerships ensuring sustained availability despite budget constraints elsewhere.
Community organizations express readiness but lack financial means required purchasing stock independently emphasizing urgent need for collaboration across all government levels moving forward if equitable access goals are truly prioritized nationwide going forward .
“This moment calls on governments working together so we can place these life-saving tools directly into hands needing them most,” stated one frontline advocate familiar with dynamics affecting marginalized populations disproportionately.”